When you’re prescribed a new medication, you might hear your doctor or pharmacist mention biosimilar or generic. Both sound like cheaper versions of brand-name drugs-but they’re not the same. Choosing the right one matters for your safety, effectiveness, and out-of-pocket costs. If you’re managing a chronic condition like rheumatoid arthritis, diabetes, or cancer, understanding the difference isn’t just helpful-it’s essential.
What’s the Real Difference Between Biosimilars and Generics?
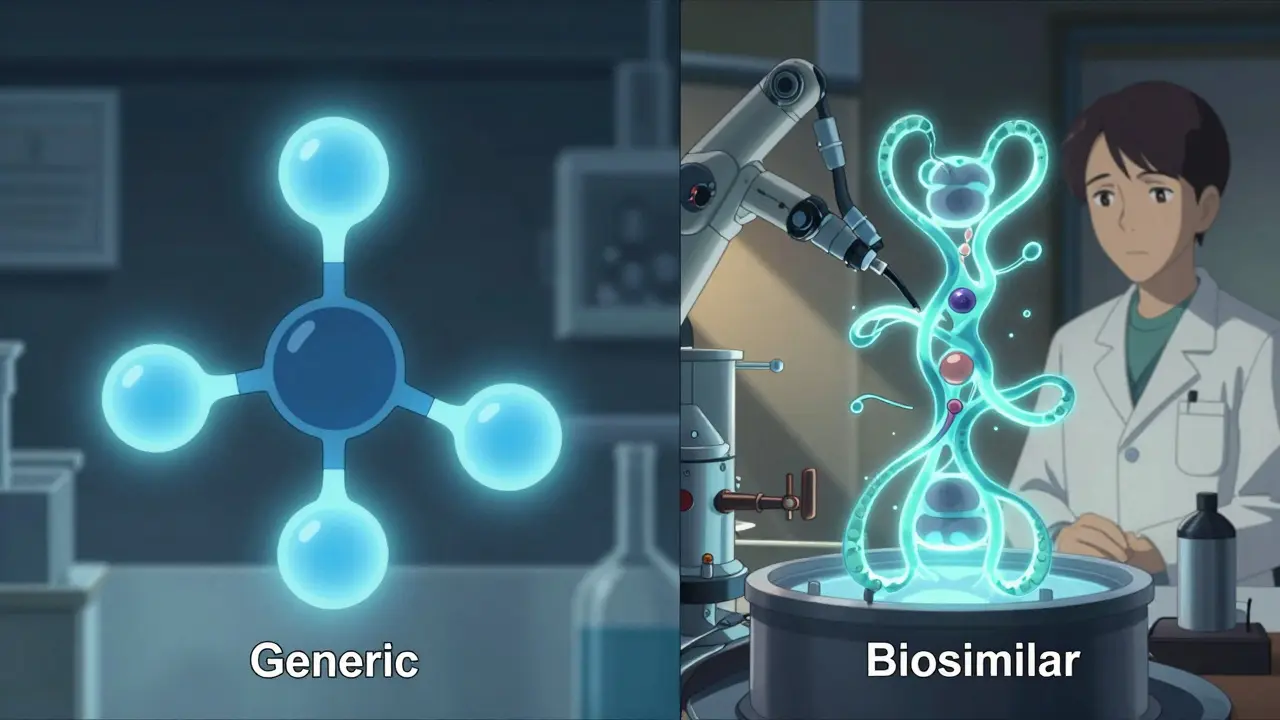
Generics are copies of small-molecule drugs. Think of them like a photocopy of a simple drawing. If the original drug is atorvastatin (Lipitor) for cholesterol, the generic is chemically identical-same atoms, same structure, same way it works in your body. They’re made in labs using precise chemical reactions. The FDA requires generics to match the brand-name drug in strength, dosage, and how quickly your body absorbs it. That’s why they’re considered interchangeable by default in most states. Biosimilars are different. They’re copies of complex biologic drugs-large proteins made from living cells, like antibodies or hormones. These aren’t simple molecules. They’re like a hand-sculpted clay statue: even if two artists use the same clay and tools, no two will be exactly alike. Biosimilars are made using living cells (often from hamster or insect cells) in controlled bioreactors. Minor differences in the manufacturing process can change the protein’s shape slightly. That’s why they’re not called “identical”-they’re “highly similar.” The FDA requires extensive testing to prove they work the same way in your body and don’t cause more side effects.Cost Savings: Generics Win Big, Biosimilars Help Too
If your goal is to save money, generics are the clear winner. They typically cost 80-85% less than the original brand-name drug. For example, a month’s supply of generic levothyroxine for thyroid issues might cost under $10, while the brand version can be $50 or more. Biosimilars save less-usually 15-20% off the price of the original biologic. That might not sound like much, but biologics are expensive. A single dose of Humira (adalimumab) can cost over $2,000. A biosimilar might bring that down to $1,600. Over a year, that’s still thousands saved. In oncology, biosimilars for drugs like trastuzumab (Herceptin) can cut treatment costs by $1,200 per infusion. The bigger picture? Generics have been around since the 1980s and are used for common conditions like high blood pressure, infections, and depression. Biosimilars are newer and mostly used for serious, chronic diseases where no small-molecule alternatives exist.How Are They Approved? It’s Not the Same Process
Generics go through a streamlined path called the Hatch-Waxman Act. The manufacturer doesn’t need to repeat full clinical trials. They just prove their drug behaves the same in the body as the original-through blood tests measuring absorption and concentration. It’s fast, cheap, and reliable. Over 11,000 generics are approved in the U.S. alone. Biosimilars follow the BPCIA pathway, created in 2010. This isn’t just a shortcut-it’s a marathon. Developers must:- Do hundreds of lab tests to compare molecular structure
- Run animal studies
- Conduct clinical trials in humans
- Prove no meaningful difference in safety or effectiveness
Can They Be Switched Automatically at the Pharmacy?
With generics, yes-almost always. In 49 states, pharmacists can swap a brand-name drug for a generic without asking your doctor, unless the prescription says “dispense as written.” It’s automatic, legal, and common. Biosimilars are trickier. Only those labeled “interchangeable” can be swapped without a doctor’s approval. As of 2024, only a handful have that status-like Semglee (insulin glargine) and Cyltezo (adalimumab). Even then, 28 states require the pharmacist to notify your doctor within 72 hours of the switch. Some states still require the prescriber to explicitly approve the switch. This matters because switching biologics can cause anxiety. Patients with autoimmune diseases often worry their symptoms will flare up. But real-world data shows otherwise. A 2022 review of 128 studies found no increase in side effects or treatment failure when patients switched from reference infliximab to its biosimilar.When Are Biosimilars the Only Option?
You can’t make a generic version of a biologic drug. Why? Because they’re too complex. You can’t chemically synthesize a 150,000-dalton antibody like you can a 500-dalton pill. So if you’re taking a drug like:- Humira (adalimumab) for psoriasis or rheumatoid arthritis
- Herceptin (trastuzumab) for breast cancer
- Enbrel (etanercept) for ankylosing spondylitis
What About Safety and Side Effects?
Generics have a 40-year track record. Studies like the 2019 JAMA analysis of 47 trials found no difference in safety or effectiveness between brand-name and generic cardiovascular drugs. The risk of side effects is the same. Biosimilars are newer, but the data is strong. The FDA’s Adverse Event Reporting System shows biosimilar infliximab has nearly identical safety rates to the original-0.12 adverse events per 100 patient-years versus 0.15 for the brand. That’s not statistically different. One concern is immunogenicity-your body reacting to the drug as if it’s foreign. This can cause allergic reactions or reduce effectiveness. But studies show this risk is no higher with biosimilars than with the original biologic. In fact, some patients report fewer reactions after switching, possibly because biosimilar manufacturers improved purification processes.Storage and Handling: A Hidden Difference
Generics are usually stable at room temperature. You can keep them in your medicine cabinet. Biosimilars? Most need refrigeration (2-8°C). They’re sensitive to heat, light, and shaking. If you’re traveling or don’t have reliable fridge access, this matters. Some biosimilars come in pre-filled pens or syringes designed for easier storage, but you still need to follow the instructions. Pharmacists and home care nurses are trained to handle this, but patients often don’t realize it. One Reddit user shared that their elderly mother accidentally left her insulin biosimilar in a warm car-and the dose didn’t work. That’s not a flaw in the drug-it’s a logistics issue.What Do Doctors and Experts Say?
The American College of Rheumatology recommends biosimilars as first-line treatment for rheumatoid arthritis. They say switching from Humira to its biosimilar doesn’t increase the risk of flare-ups. The American Society of Clinical Oncology encourages biosimilars in cancer care, calling them a “significant opportunity to reduce costs without compromising outcomes.” But not all doctors are confident. A 2023 AMA survey found only 58% of non-specialist physicians felt “very confident” prescribing biosimilars. Many haven’t been trained on the science behind them. That’s changing. The FDA’s Biosimilars Community has grown to over 25,000 registered professionals since 2021. Educational resources are now widely available.
Real Patient Stories
A patient with Crohn’s disease on PatientsLikeMe wrote: “I switched to the infliximab biosimilar. My symptoms stayed under control, and my copay dropped from $400 to $80. My GI doctor explained the science. That made all the difference.” Another, with HER2+ breast cancer, shared: “My oncologist switched me from Herceptin to the biosimilar. My tumor markers didn’t change. I saved $1,200 per infusion. No side effects. I wish I’d known sooner.” But not everyone is comfortable. A 2022 National Psoriasis Foundation survey found 42% of patients were worried about biosimilar effectiveness. 28% refused to switch at first. Education helped-once they understood the data, most changed their minds.What Should You Do?
If you’re prescribed a new drug:- Ask: Is this a biologic or a small-molecule drug?
- If it’s small-molecule (pill, capsule), ask if a generic is available. It’s almost always the best choice.
- If it’s a biologic (injection, IV), ask if a biosimilar is an option. Don’t assume it’s inferior.
- Check your insurance formulary. Many plans now require biosimilars before covering the brand.
- Ask your pharmacist to explain the switch. If you’re nervous, request a trial period.
- Keep track of symptoms. If something changes after switching, tell your doctor-but don’t assume it’s the drug. Stress, diet, or infections can also cause flare-ups.
The Bottom Line
Biosimilars and generics aren’t the same-but they’re both safe, effective, and meant to save you money. Generics are the go-to for everyday medications. Biosimilars are your lifeline to affordable biologics for serious conditions. The science is solid. The savings are real. And the medical community agrees: choosing either one isn’t settling. It’s smart.Are biosimilars as safe as brand-name biologics?
Yes. Every biosimilar approved by the FDA must prove it has no clinically meaningful differences in safety, purity, or potency compared to the original biologic. Real-world data from over 38,000 patients shows no increase in side effects or treatment failure after switching. The FDA monitors adverse events closely, and biosimilars have safety profiles nearly identical to their reference products.
Can I switch from a brand-name drug to a biosimilar without my doctor’s permission?
Only if the biosimilar is labeled “interchangeable” and your state allows automatic substitution. As of 2024, only a few biosimilars have that status, like Cyltezo (for Humira) and Semglee (for Lantus). Even then, 28 states require the pharmacist to notify your doctor within 72 hours. Always check your state’s laws and ask your pharmacist before any switch.
Why are biosimilars more expensive than generics?
Because they’re far more complex to make. Generics are chemically synthesized and can be replicated exactly. Biosimilars are made from living cells, which introduces tiny variations. Developing a biosimilar requires hundreds of lab tests, animal studies, and clinical trials-costing $100-250 million and taking 8-10 years. Generics cost $2-3 million and take 3-4 years. That’s why biosimilars save 15-20%, not 80-85% like generics.
Do I need to get blood tests after switching to a biosimilar?
Not routinely. If you’re on a biologic for a condition like rheumatoid arthritis or Crohn’s, your doctor will monitor your disease activity regardless of whether you’re on the brand or biosimilar. Blood tests are done to track your condition-not because biosimilars are riskier. Some doctors may check drug levels in rare cases, but this isn’t standard practice for approved biosimilars.
What if I have an allergic reaction after switching?
Report it immediately to your doctor and pharmacist. Allergic reactions can happen with any drug, brand-name or biosimilar. The FDA tracks these through its Adverse Event Reporting System. If a pattern emerges, they investigate. But current data shows no higher risk with biosimilars. Many patients report fewer reactions after switching-possibly due to improved purification methods in newer manufacturing.
Are biosimilars covered by Medicare?
Yes. Since the Inflation Reduction Act of 2022, Medicare Part B no longer penalizes providers for using biosimilars. This has increased their use in clinics and hospitals. Most Medicare Advantage plans also cover biosimilars with low copays. If you’re on Medicare and prescribed a biologic, ask if a biosimilar is available-it could cut your out-of-pocket costs significantly.
Can I switch back to the brand-name drug if I’m not happy with the biosimilar?
Yes. You can always ask your doctor to switch you back. Insurance may require you to try the biosimilar first, but if you experience side effects, lack of effectiveness, or just feel uncomfortable, your doctor can request a prior authorization for the brand. Many patients switch back successfully. The key is communication-don’t suffer in silence.

Comments
Nicole Rutherford December 17, 2025 AT 12:28
Ugh, I still can't believe some pharmacies just swap out my biologic without telling me. My flare-up last month? Totally because they switched me to some biosimilar I didn't ask for. Doctors don't get it - this isn't aspirin.
And don't even get me started on how they act like it's 'the same.' It's not. My body knows the difference.
Mark Able December 18, 2025 AT 00:23
Bro I switched to the Humira biosimilar last year and my copay went from $500 to $90. I was scared too but my rheumatologist showed me the FDA data - zero difference in labs or symptoms. I even saved enough to take a trip to Bali. Life’s too short to overpay for meds.
Also, my dog loves the new pen - it’s less scary to inject than the old vial. Weird flex but true.
Chris Clark December 18, 2025 AT 12:05
So like, generics are just chemical copies, right? Like, same molecules, same everything. Biosimilars are more like… a cousin of the original? Made from living cells so it’s never gonna be *exactly* the same, but close enough that your immune system doesn’t throw a fit.
Also, the storage thing is wild - my cousin left her insulin biosimilar in a hot car and it went bad. Like, it’s not a pill, it’s a protein. You can’t just toss it in the glovebox.
Also, the cost difference? Yeah, biosimilars ain’t cheap, but they’re still a godsend when you’re paying $2k per shot. I’d rather save $400 than $1800, honestly.
Dorine Anthony December 20, 2025 AT 09:13
Just wanted to say I switched to the biosimilar for my Crohn’s meds last year and honestly? Nothing changed. No flare-ups, no weird side effects. My insurance pushed me to it, and I was nervous, but my GI doc explained everything. Turns out, the science is legit.
Also, the $80 copay vs $400? Yeah, that’s the real win. No drama, just better access.
William Storrs December 21, 2025 AT 04:13
You got this. Seriously. Switching to a biosimilar isn’t settling - it’s upgrading your life. Less stress over bills, more energy to live. I know it feels scary, but you’re not losing quality - you’re gaining freedom.
And if you’re worried? Talk to your pharmacist. Ask for a trial. Most places let you switch back if you’re not feeling it. You’ve got options. You’re not alone.
James Stearns December 22, 2025 AT 10:17
It is a matter of profound concern that regulatory agencies have permitted the dissemination of biopharmaceutical products that, while deemed "highly similar," are not chemically identical to their reference agents. The implications for patient safety, particularly in immunocompromised populations, remain inadequately characterized in longitudinal studies.
Moreover, the notion that cost reduction equates to therapeutic equivalence is a fallacy rooted in economic reductionism, not clinical science. One must question the integrity of a system that prioritizes fiscal efficiency over biological fidelity.
Nina Stacey December 22, 2025 AT 12:44
I switched to the biosimilar and honestly I felt weird for like a week but then I got used to it and my skin stopped being so flaky and my insurance paid like 90 percent so I’m not complaining I mean I was scared at first but my doctor said it was fine and now I’m like why did I wait so long also my cat licked my injection pen once and I thought I was gonna die but nothing happened so maybe it’s all good idk
also can you believe they make these in hamster cells like what even is that
Dominic Suyo December 22, 2025 AT 14:46
Oh wow, so we’re just gonna hand-wave away the fact that biosimilars are made in *living cells* and call it "safe"? That’s like saying two hand-sculpted clay statues are interchangeable because they’re both brown.
And the FDA’s data? Please. They’re funded by pharma. You think they’re gonna flag a biosimilar that causes a slow-burn autoimmune reaction five years down the line? Nah. They’ll call it "coincidental."
Meanwhile, your $1,600 injection is still more than my rent. Welcome to the future of healthcare - cheaper, shakier, and full of invisible trade-offs.
Kevin Motta Top December 23, 2025 AT 23:09
Generics = pill. Biosimilars = injection made from living cells. Same goal, totally different science.
Save money? Yes. Don’t panic? Also yes.
Alisa Silvia Bila December 25, 2025 AT 10:49
My mom was terrified to switch from Herceptin to the biosimilar, but after she did, her tumor markers stayed flat and she saved $1,000 per round. She said the hardest part was the fear, not the medicine.
It’s okay to be nervous. But don’t let fear stop you from getting the care you need - especially when the data says it works.
And honestly? If your insurance makes you switch, you’re not losing out. You’re winning time, money, and peace of mind.
Marsha Jentzsch December 26, 2025 AT 08:14
Wait wait wait - so you’re telling me they’re making these biosimilars in HAMSTER CELLS?!?!? And you’re just supposed to trust that?! What if they’re secretly mutated?! What if the FDA is being paid off by Big Pharma?!
I read on a blog that one guy got a rash and then his dog died and then his WiFi stopped working - it’s all connected!!!
Also, why do they need to refrigerate it?! That’s a sign! That’s a sign they’re not real!!!
I’m not switching. I’m not. I’m sticking with the brand. Even if it costs my retirement.
Janelle Moore December 28, 2025 AT 05:30
They didn’t even tell me I was switched to the biosimilar until I got the bill. I asked my doctor and he said "it’s the same." But I know my body. I felt different. Now I’m paranoid every time I get an injection.
And why do they keep saying "clinical trials prove it’s safe"? Who funded those trials? The same company that makes the original? That’s not science. That’s marketing.