When you’re taking a new medication or joining a clinical trial, you might see the term serious adverse event in your paperwork. It sounds scary. And it should - because it’s meant to flag real risks. But here’s the thing most people miss: serious doesn’t mean severe. And that difference could save you from unnecessary panic - or help you spot a real danger you didn’t know to watch for.
What the FDA Actually Means by ‘Serious’
The U.S. Food and Drug Administration (FDA) doesn’t use ‘serious’ the way you might think. It’s not about how bad the symptom feels. It’s about what happens because of it. According to FDA rules, an adverse event is classified as serious if it leads to one of five specific outcomes:- Death - even if it’s just suspected to be linked to the drug or device
- Life-threatening - meaning you were in danger of dying at the time the event happened
- Hospitalization - either you had to be admitted, or your stay got longer by at least 24 hours
- Permanent disability or damage - something that stops you from doing normal daily activities
- A birth defect - if you’re pregnant and the medicine affects the baby
There’s also something called an ‘Important Medical Event’ - a situation that doesn’t quite hit those five points but could still put you at risk if left unchecked. For example, a sudden drop in blood pressure that didn’t land you in the hospital but required emergency IV fluids. That counts too.
This system isn’t just paperwork. In 2022 alone, it led to 128 safety alerts and 47 updates to drug labels. That’s how the FDA catches hidden dangers after a drug is already on the market.
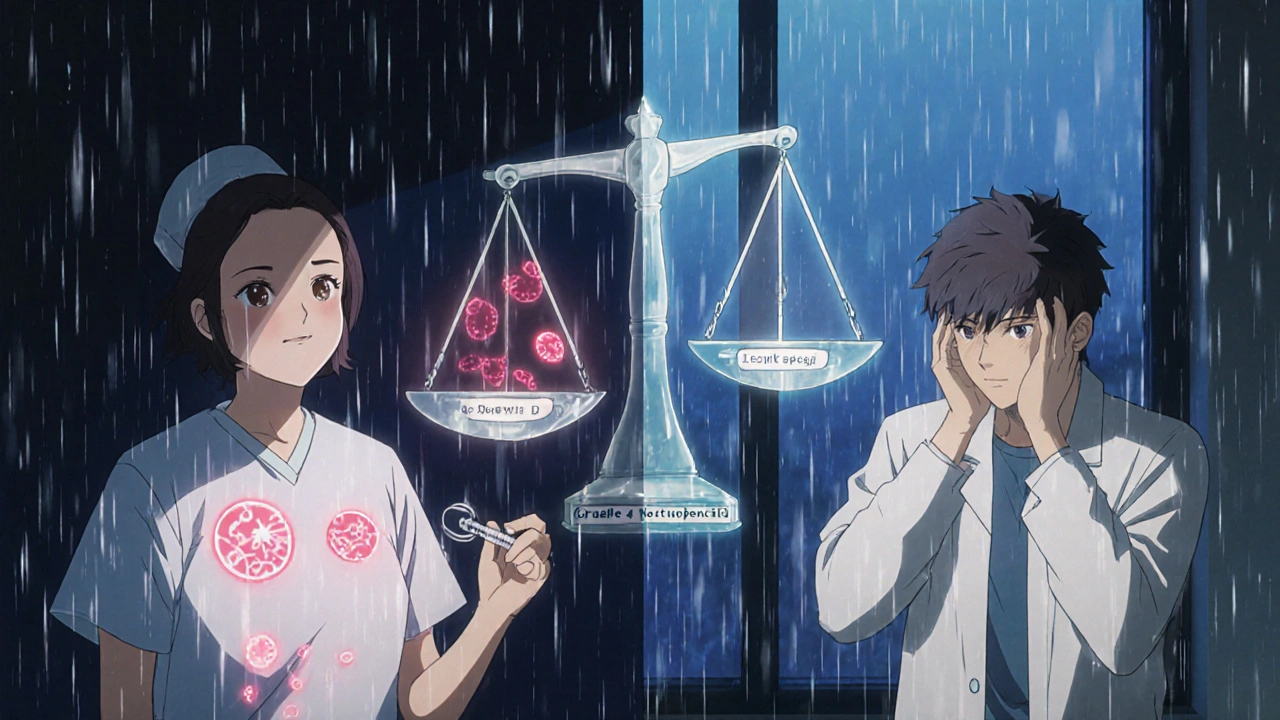
Why ‘Serious’ and ‘Severe’ Are Not the Same
This is where most patients get confused.Doctors and trial forms often use a scale called CTCAE to rate how severe a side effect is. That scale goes from Grade 1 (mild) to Grade 5 (fatal). A Grade 3 event is considered ‘severe’ - maybe you’re vomiting nonstop, your white blood cell count crashed, or your liver enzymes spiked. But if none of those symptoms led to hospitalization, permanent harm, or life-threatening danger, the FDA doesn’t call it ‘serious.’
Here’s a real example: In cancer trials, 68% of Grade 3 or 4 side effects (like low blood counts or nausea) were not labeled as serious because they were expected, reversible with treatment, and didn’t require hospital stays. But patients who didn’t know the difference panicked. One patient on the Inspire forum said, ‘I thought Grade 4 neutropenia meant I was dying - until my nurse explained it was normal for this drug and could be fixed with a shot.’
On the flip side, a mild headache that triggers a seizure? That’s not severe by the scale - but it’s serious because it could kill you. The FDA’s system catches those hidden risks.
How This Affects You as a Patient
If you’re reading a medication guide or a clinical trial consent form, look for the section titled ‘Warnings and Precautions.’ That’s where drugmakers list the serious adverse events they’ve seen in studies. They’ll say things like: ‘Serious infections occurred in 2.3% of patients.’ That’s not a guess - it’s based on data collected using FDA’s strict rules.But here’s the catch: most people don’t understand what that number means. A 2022 survey of 1,543 trial participants found that 78% couldn’t tell the difference between ‘serious’ and ‘severe.’ That confusion led to unnecessary stress - and sometimes, people dropped out of trials they could’ve safely completed.
One patient with Type 1 diabetes said this clarity changed everything: ‘Knowing that hospitalization for diabetic ketoacidosis counted as a serious event helped me know when to call 911 during the trial.’ That’s the power of understanding the definition.
What You Should Do When You See ‘Serious Adverse Event’
Don’t ignore it. Don’t panic. Do this:- Find the exact event - Is it listed as ‘liver failure’? ‘Heart attack’? ‘Uncontrolled bleeding’? Write it down.
- Ask: Did this cause hospitalization, disability, or threaten my life? If yes, it’s serious - even if it feels ‘mild’ at first.
- Check the frequency - Was it seen in 1 out of 100 people? Or 1 out of 10,000? That tells you how likely it is.
- Ask your provider: ‘Is this something I should watch for at home?’ Some serious events are rare but need immediate action. Others are monitored in the clinic.
For example, if you’re on a new diabetes drug and you get a sudden, severe stomach ache that won’t go away - even if you’re not vomiting - ask: Could this be pancreatitis? That’s a serious event. You don’t wait for it to get worse. You call your doctor.
Reporting Events Yourself - And Why It Matters
The FDA doesn’t hear about every bad reaction. Studies show only 1% to 10% of adverse events get reported - mostly by doctors and hospitals. Patients rarely do.But you can. The FDA’s MedWatch program lets you report side effects directly using Form 3500B. In 2022, they received over 38,000 reports from patients - up 12% from the year before. That’s not just data. It’s a safety net.
One patient reported a rare heart rhythm issue after starting a new cholesterol drug. Two months later, the FDA added a warning to the label. That patient didn’t know they’d helped protect others. But they did.

What’s Changing - And What’s Coming
The FDA knows patients are confused. That’s why they’re making changes:- By 2025, all clinical trial websites must include a plain-language summary of serious adverse events - no jargon.
- A new patient portal is launching in late 2024 to explain SAEs in simple terms, with real examples.
- AI tools are now helping FDA reviewers spot dangerous patterns faster - cutting review time from 30 days to 7 for the most urgent cases.
These aren’t just tech upgrades. They’re about giving you the power to understand what’s happening to your body - without needing a medical degree.
Bottom Line: Know the Difference, Stay in Control
A serious adverse event isn’t about how much pain you’re in. It’s about whether your health took a dangerous turn - one that could cost you your life, your mobility, or your future. The FDA’s system is designed to catch those moments early. But it only works if you know what to look for.Next time you see ‘serious adverse event’ on a form, pause. Ask: ‘Did this hospitalize me? Make me permanently worse? Almost kill me?’ If the answer is yes - it matters. If not - it might still be uncomfortable, but it’s not a red flag.
Understanding this isn’t about fear. It’s about control. You’re not just a patient. You’re a partner in your own safety. And that starts with knowing what the words really mean.
Is a serious adverse event the same as a side effect?
All serious adverse events are side effects, but not all side effects are serious. A side effect is any unwanted reaction to a drug - even a mild headache. A serious adverse event is a side effect that leads to death, hospitalization, life-threatening danger, permanent damage, or a birth defect. Think of it this way: a side effect is the symptom; a serious adverse event is the dangerous outcome.
If I had a bad reaction but didn’t go to the hospital, is it still serious?
Maybe. The FDA includes ‘Important Medical Events’ - situations that didn’t meet the five standard criteria but still needed urgent treatment to prevent serious harm. For example, if you had a sudden drop in blood pressure that required IV fluids in an urgent care clinic, that could be classified as serious. If you’re unsure, report it. The FDA encourages reporting even borderline cases.
Why do some drug labels say ‘serious side effects’ while others say ‘severe’?
Drug companies are required to use ‘serious’ when referring to FDA-defined outcomes. ‘Severe’ is often used informally to describe how intense a symptom feels. If a label says ‘severe nausea,’ it means it was intense - but not necessarily serious. Always check if the label links it to hospitalization, death, or permanent damage. If it doesn’t, it’s not a serious event by FDA standards.
Can a serious adverse event happen after I stop taking the drug?
Yes. Some serious events show up days or even weeks after stopping a medication. For example, liver damage from certain antibiotics can appear after treatment ends. That’s why clinical trials track patients for months after the last dose. If you develop new symptoms after stopping a drug - especially if they’re sudden or worsening - tell your doctor and consider reporting it to MedWatch.
How do I report a serious adverse event as a patient?
Go to the FDA’s MedWatch website and download Form 3500B. You can fill it out online or print it. You’ll need: the name of the drug or device, the event you experienced, when it happened, and your contact info. You don’t need a doctor’s note. The FDA accepts reports from anyone - patients, family members, caregivers. In 2022, over 38,000 reports came from patients like you. Your report could help someone else avoid the same risk.


Comments
Linda Rosie November 23, 2025 AT 15:29
This is incredibly clear. Finally, someone explained the difference between serious and severe without jargon.
Thank you.
Vivian C Martinez November 25, 2025 AT 10:57
I wish every clinical trial form came with this level of clarity. So many patients panic over Grade 3 side effects that are completely manageable. This post could prevent a lot of unnecessary stress.
Ross Ruprecht November 25, 2025 AT 22:59
Ugh another wall of text. Can we just say 'if it lands you in the ER it's serious' and be done with it?
Bryson Carroll November 26, 2025 AT 21:04
The FDA system is a joke really
they classify everything based on bureaucratic checkboxes not actual patient experience
my cousin got liver damage from a drug and they didn't flag it because he didn't get hospitalized for 24 hours straight
what a farce
Lisa Lee November 28, 2025 AT 20:08
This is why American healthcare is broken. We turn everything into a legal formality instead of just telling people the truth. In Canada we just say 'this might kill you' and move on.
Jennifer Shannon November 29, 2025 AT 10:18
You know, I've been thinking about this for weeks now... it's not just about the FDA's definitions, it's about how we've culturally detached ourselves from the language of our own bodies.
We've been trained to see medical terms as something that belongs to doctors, not to us.
But when you realize that 'serious' doesn't mean 'scary' and 'severe' doesn't mean 'dangerous' - it's like unlocking a secret code that your body has been whispering to you all along.
That moment when you stop reading a label like it's a foreign poem and start reading it like a conversation with your own health... that's when the real power begins.
It's not about fear. It's about reclamation.
And honestly? That's the most revolutionary thing this post does.
Suzan Wanjiru November 29, 2025 AT 21:57
The Important Medical Event part is so underdiscussed
like I had a BP drop that needed IV fluids at urgent care but no hospital stay
they told me it wasn't serious but I reported it anyway
turns out 3 others had the same thing
now the label got updated
Kezia Katherine Lewis December 1, 2025 AT 09:16
The terminology alignment between CTCAE and FDA-defined serious adverse events remains a persistent interface gap in translational research communication. Patients often conflate ordinal severity grading with regulatory classification thresholds, leading to suboptimal risk perception and decisional inertia.
Henrik Stacke December 1, 2025 AT 09:26
I must say, this is one of the most lucid explanations I’ve ever encountered on this subject. Truly, the distinction between ‘serious’ and ‘severe’ is not merely semantic-it is existential. In the UK, we’ve seen too many cases where patients withdrew from life-saving trials due to misinterpretation. This clarity? It’s not just helpful. It’s heroic.
Manjistha Roy December 2, 2025 AT 22:22
This should be mandatory reading for every patient starting any new medication or trial. I've seen too many people quit treatment because they thought a bad headache meant they were dying. Knowledge is power, and this post gives people real power.
Jennifer Skolney December 3, 2025 AT 11:37
I’m so glad someone finally explained this! 😊 My mom was terrified of her new blood pressure med because the form said ‘serious adverse event: dizziness’ - she thought it meant she’d collapse. Turns out it was just mild lightheadedness that went away in a week. This post would’ve saved her so much anxiety.
JD Mette December 3, 2025 AT 18:30
I read this while waiting for my appointment. It made me feel less alone. I’ve had two reactions that weren’t flagged as serious but scared me. Now I know I wasn’t overreacting - I just needed better info.
Olanrewaju Jeph December 5, 2025 AT 04:26
This is exactly the kind of information that should be translated into local languages and shared in community clinics. In Nigeria, many patients assume all side effects are dangerous because no one explains the difference. This post is a gift.
Dalton Adams December 6, 2025 AT 03:17
You people are missing the real point. The FDA doesn't care about patients - they care about lawsuits and liability. That’s why they define 'serious' the way they do. It's not to protect you, it's to protect Big Pharma. The fact that you're thanking them for this 'clarity' is the real tragedy. 😏